
Teen Vaccine Trial Volunteer Kearston Stepenosky
The trial of the century.
-
CategoryHealth
-
Written byJean Trinh
-
Photographed byShane O’Donnell
 When Kearston Stepenosky walked into the National Research Institute last October to receive her first dose of the Pfizer-BioNTech COVID-19 vaccine, she recalls being “by far the youngest person there.”
When Kearston Stepenosky walked into the National Research Institute last October to receive her first dose of the Pfizer-BioNTech COVID-19 vaccine, she recalls being “by far the youngest person there.”
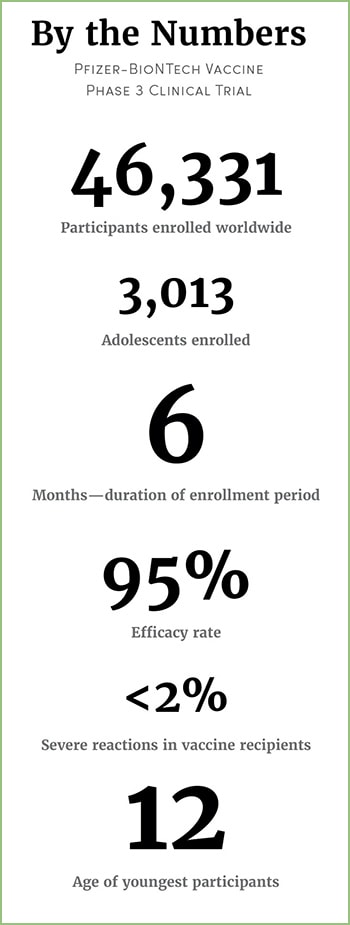
The Calabasas High School junior and basketball team captain was only 16 at the time when she volunteered to be part of the pharmaceutical companies’ phase 3 clinical study. Initially, the trial was composed of participants 18 and older, but last September, it expanded to adolescents as young as 16, and the following month to those 12 to 15 years old.
“As soon as they started to allow minors in, I knew I wanted to be a part of it,” says Kearston, who’s now 17.
Kearston says she has always been fascinated with medicine, an interest that grew when she studied bacteriophage therapy as part of her school’s AP Capstone program. She was also inspired by her father, Dan Stepenosky, superintendent of the Las Virgenes Unified School District. He participated in the Pfizer trials last August.
“I just remember thinking how interesting it was and how much I wanted to be part of it and help in that small way that I could,” Kearston shares. “I know it’s something that feels like just a tiny, tiny statistic, but obviously, people need to get involved.”
Dan, a colon cancer survivor, had similar reasons for volunteering. “I’m the superintendent of a school district. Obviously, we want our staff and students to be safe. We want to open up our schools and have kids on campus. I like to solve stuff and move forward.”
However, it took some convincing to get Kearston’s parents on board regarding her getting the vaccine. Describing his daughter as a “force of nature,” Dan says she persistently asked him and his wife, Sharon, for 10 days straight if she could participate in the study.
 “I’m 52, so it’s one thing for me to be involved in something experimental,” Dan says. “But when you’re 16, you’re still growing and hopefully have a long, wonderful life ahead of you. I didn’t want to have any kind of medical complications that would impact that. So I was nervous about it.”
“I’m 52, so it’s one thing for me to be involved in something experimental,” Dan says. “But when you’re 16, you’re still growing and hopefully have a long, wonderful life ahead of you. I didn’t want to have any kind of medical complications that would impact that. So I was nervous about it.”
After seeing how passionate Kearston felt about participating, the family discussed it with doctors and then gave it the green light.
Leading up to her first shot, Kearston went through phone screenings, got a physical exam and blood work done, and took a COVID-19 test. She says she wasn’t nervous because Pfizer outlined the risks carefully and was extremely communicative about them. Kearston had also seen her father go through it, and he didn’t have much of a reaction, except for brief joint and muscle soreness.
On the morning of October 22, Kearston, accompanied by her father, went in to get her first dose. For the following five days, she had chills and felt cold-like symptoms, though she says they weren’t severe. When she received her second vaccine on November 5, she had similar symptoms, but they lasted only a day.
“It’s really far more important that the greater population be vaccinated so everything can get back to normal, because safety is much more important than that minor reaction I had,” Kearston says.
As part of the trial, Kearston will be monitored for a total of 26 months. She fills out a health questionnaire weekly and goes in to get an examination every couple months. She hasn’t had any negative reactions since her last shot.
In December, when the results of the Pfizer trial revealed the vaccine to be 95% effective, Dan says he and Kearston did a happy high five because they “bet on the right horse.”
Dan admits it might sound strange, but he found it to be a great father-daughter experience to go through the trial together. “She’ll tell her children and her grandchildren about it,” he says. “She’ll say, ‘My dad was so crazy he let me try out for a vaccine for that global pandemic from 50 years ago.’ So, it was a cool thing to do together to try and help.”
The Building Boom in the Valley for Senior Living Communities Has Escalated
Upscale livin’ for active retirees.











